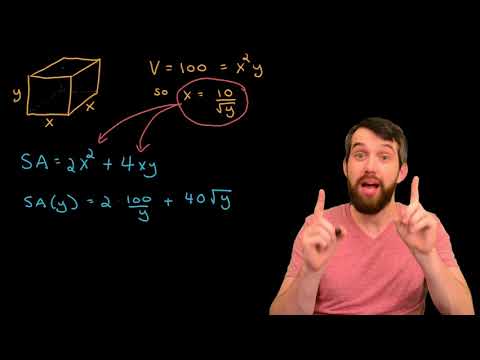Shows the evolution in volume-average diameter for the growing latex particles during the TFEMA polymerization as determined by in situ SAXS studies using the stirrable reaction cell. For the surfactant-free formulation, two distinct regimes are observed after nucleation (see Figure Figure8 8a). Thereafter, there is a brief increase in the rate of particle growth, which then slows down until the TFEMA polymerization is more or less complete, producing colloidally stable latex particles with a volume-average particle diameter of 353 ± 9 nm after 80 min. Bearing in mind the effect of polydispersity, this final particle size is reasonably consistent with the volume-average particle diameter of 444 nm determined by post-mortem DLS analysis. Furthermore, the evolution in particle diameter determined by in situ SAXS (Figure Figure8 8a) is similar to that indicated by DLS studies of the equivalent laboratory-scale synthesis (Figure Figure4 4a). For example, mean diameters of the nascent particles observed after 8 min are 88 and 94 nm for the SAXS and DLS data, respectively.
The rate of particle growth begins to slow down after approximately 40 min for both the SAXS and equivalent laboratory-scale syntheses. At this time point, the mean particle diameters are 312 and 413 nm, respectively. During the early stages of the TFEMA polymerization (8–27 min), the rate of particle growth is constant.
Both in situ SAXS and DLS studies suggest a brief increase in the rate of growth after approximately 27 min. In principle, this feature should correspond to the end of Interval II (Figure Figure1 1b). Since there are no remaining monomer droplets, polymerization proceeds under monomer-starved conditions, which explains the slower rate of particle growth observed after 30 min. The solution conductivity was also monitored in situ during the aqueous emulsion polymerization of TFEMA using the surfactant-free formulation, see Figure S5. In this case, there is no measurable solution conductivity for the first 28 min of the TFEMA polymerization, at which point the instantaneous monomer conversion is approximately 60%, see Figure Figure4 4a.
Subsequently, there is a dramatic increase in solution conductivity in the 29–31 min interval. Interestingly, this time point corresponds to a discernible inflection point during the evolution in particle size indicated by DLS studies during the equivalent laboratory-scale synthesis, see Figure Figure4 4a. Moreover, optical microscopy studies confirm essentially full consumption of the monomer droplets after approximately 30 min, see Figure S7. Thus, it seems likely that this time point corresponds to the Interval II/Interval III boundary as shown in Figure Figure8 8a. Thereafter, the rate of increase in solution conductivity is reduced, with a maximum solution conductivity of 640 μS cm–1 being observed after 55 min followed by a gradual reduction in conductivity to a limiting value of 590 μS cm–1 after 75 min. According to the NMR kinetic data in Figure Figure4 4a, this latter time point corresponds to the end of the polymerization.
The TFEMA polymerization kinetics are monitored using 1H NMR spectroscopy, while postmortem TEM analysis confirms that the final nanocomposite particles possess a well-defined core–shell morphology. Nucleation occurs after 10–15 min and the nascent particles quickly become swollen with TFEMA monomer, which leads to a relatively fast rate of polymerization. Additional surface area is created as these initial particles grow and anionic silica nanoparticles adsorb at the particle surface to maintain a relatively high surface coverage and hence ensure colloidal stability. At high TFEMA conversion, a contiguous silica shell is formed and essentially no further adsorption of silica nanoparticles occurs. A population balance model is introduced into the SAXS model to account for the gradual incorporation of the silica nanoparticles within the nanocomposite particles. The persulfate-initiated aqueous emulsion polymerization of 2,2,2-trifluoroethyl methacrylate is studied by time-resolved small-angle X-ray scattering at 60 °C using a stirrable reaction cell.
TFEMA was preferred to styrene because it offers much greater X-ray scattering contrast relative to water, which is essential for sufficient temporal resolution. The evolution in particle size is monitored by both in situ SAXS and ex situ DLS in the absence or presence of an anionic surfactant . Post-mortem SAXS studies confirmed the formation of well-defined spherical latexes, with volume-average diameters of 353 ± 9 nm and 68 ± 4 nm being obtained for the surfactant-free and SDS formulations, respectively. 1H NMR spectroscopy studies of the equivalent laboratory-scale formulations indicated TFEMA conversions of 99% within 80 min and 93% within 60 min for the surfactant-free and SDS formulations, respectively. Comparable polymerization kinetics are observed for the in situ SAXS experiments and the laboratory-scale syntheses, with nucleation occurring after approximately 6 min in each case. After nucleation, scattering patterns are fitted using a hard sphere scattering model to determine the evolution in particle growth for both formulations.
Moreover, in situ SAXS enables identification of the three main intervals that are observed during aqueous emulsion polymerization in the presence of surfactant. These intervals are consistent with those indicated by solution conductivity and optical microscopy studies. Significant differences between the surfactant-free and SDS formulations are observed, providing useful insights into the mechanism of emulsion polymerization. Initially, most of this surfactant is either present in the form of micelles or is adsorbed at the surface of the monomer droplets. At the end of the polymerization, the majority of the surfactant is adsorbed at the surface of the final latex particles. At intermediate monomer conversions, the solution conductivity—which can be readily monitored in situ—depends on the relative populations of monomer droplets, micelles, free surfactant, and growing latex particles.
Once particle nucleation has occurred, free surfactant molecules adsorb onto the growing nascent particles to confer anionic surface charge and hence colloidal stability. This depletion of free surfactant leads to a gradual reduction in conductivity and the period between 0 and 11 min corresponds to Interval I (Figure Figure1 1a).49 At the end of Interval I, there is no more molecularly dissolved surfactant in the aqueous continuous phase. A local maximum in conductivity is observed after approximately 28 min.
This period corresponds to the onset of Interval III. At this point, essentially all the monomer droplets have been consumed, so the remaining monomer is mainly located within the growing PTFEMA latex particles . Any free surfactant molecules remaining within the aqueous phase adsorb onto the latex particles during the latter stages of their growth, which accounts for the gradual reduction in solution conductivity observed over the following 32 min. After 60 min, the conductivity remains constant, suggesting that the TFEMA polymerization is complete. Immediately after the nucleation event, there is an initial period of rapid particle growth with a concomitant increase in the number of silica nanoparticles located within the shell. After approximately 60 min (50% TFEMA conversion) the rate of particle growth is reduced and the number of silica nanoparticles within the shell remains relatively constant.
A final silica shell thickness of 20 nm is calculated, which is consistent with approximate monolayer coverage of the latex cores by the silica nanoparticles. 1H NMR spectroscopy studies indicate an overall TFEMA conversion of 96%, while SAXS analysis indicates a final volume-average core–shell particle diameter of 216 nm and a silica aggregation efficiency of approximately 75%, which are consistent with postmortem DLS, TEM and TGA studies. The latter anionic nanoparticles form a particulate shell at the surface of the PTFEMA latex cores and hence confer colloidal stabilization. Schematic cross-section of the bespoke stirrable reaction cell used. The volume of the reaction solution within this cell is approximately 2.0 mL, which is sufficient to enable postmortem analysis of the final nanocomposite particles after performing time-resolved small-angle X-ray scattering experiments. The persulfate-initiated aqueous emulsion polymerization of TFEMA at 60 °C leads to the formation of well-defined spherical latex particles when performed either under surfactant-free conditions or in the presence of SDS surfactant.
This semifluorinated vinyl monomer was preferred to styrene because it ensures much stronger X-ray contrast for the corresponding latex particles relative to water. Nucleation and subsequent particle growth has been monitored in situ for both formulations utilizing a stirrable reaction cell to perform time-resolved SAXS studies. This cell has a reaction solution volume of approximately 2.0 mL, which is sufficient to allow post-mortem analysis of the final latex particles by 1H NMR spectroscopy, DLS, and TEM. Schematic cross-section of the stirrable reaction cell used for time-resolved small-angle X-ray scattering studies of such formulations.
The volume of the reaction solution within this cell is approximately 2.0 mL, which is sufficient to enable post-mortem analysis using 1H NMR spectroscopy, TEM, and dynamic light scattering. In particular, time-resolved SAXS measurements indicate that particle nucleation occurs within 10–15 min of polymerization. This enables the nanocomposite particle diameter, silica shell thickness, mean number of silica nanoparticles within the shell, silica aggregation efficiency and packing density within the silica shell to be monitored during the TFEMA polymerization.
Formation of diblock copolymer spheres, worms and vesicles during reversible addition–fragmentation chain transfer aqueous dispersion polymerization of 2-hydroxypropyl methacrylate at 70 °C using a poly steric stabilizer. 1H NMR spectroscopy indicates more than 99% HPMA conversion within 80 min, while transmission electron microscopy and dynamic light scattering studies are consistent with the final morphology being pure vesicles. Furthermore, the change in vesicle diameter and membrane thickness during the final stages of polymerization supports an 'inward growth' mechanism. Time curve obtained from 1H NMR spectroscopy studies for the laboratory-scale aqueous emulsion polymerization of TFEMA in the presence of a glycerol-functionalized silica sol using a cationic azo initiator at 60 °C targeting 10% w/w solids.
Evolution in volume-average particle diameter and polydispersity determined by dynamic light scattering studies of aliquots periodically extracted from the quenched reaction solution (diluted to 0.20% w/w prior to analysis using deionized water). Prior to polymerization, the hydrophobic monomer mainly resides in the monomer droplets, with a relatively small fraction solubilized within surfactant micelles and a further fraction dissolved within the aqueous continuous phase. Free radicals derived from the water-soluble initiator polymerize monomer dissolved in the aqueous phase to form oligomeric radicals. To maintain colloidal stability, the remaining surfactant micelles undergo dissociation to supply additional surfactant and hence ensure monolayer coverage of the surface of the growing polymer particles.
Furthermore, the surfactant molecules that act as an emulsifier desorb from the monomer droplets to coat these particles. Once there are no remaining surfactant micelles, particle nucleation (i.e., Interval I) is complete, see Figure Figure1 1a. Thereafter, the number of latex particles remains relatively constant. Polymerization continues primarily within monomer-swollen particles with monomer droplets serving as reservoirs to supply the growing particles with further monomer . This particle growth stage (Interval II, Figure Figure1 1b) is complete when there are no remaining monomer droplets. This leads to so-called "monomer-starved" conditions and the polymerization proceeds at a slower rate until all the monomer is consumed (Interval III, Figure Figure1 1c).
Small-angle X-ray scattering studies during the formation of vinyl polymer/silica colloidal nanocomposite particles, see Fig. More specifically, such colloidal nanocomposite syntheses involve the surfactant-free aqueous emulsion polymerization of 2,2,2-trifluoroethyl methacrylate in the presence of an ultrafine glycerol-functionalized aqueous silica sol. The proposed mechanism of formation of nanocomposite particles during aqueous emulsion polymerization of a water-immiscible monomer in the presence of silica nanoparticles. Addition of AIBA leads to electrostatic adsorption of some of this cationic initiator onto the anionic silica nanoparticles, with the rest remaining in the aqueous continuous phase.
Surface polymerization of TFEMA produces hydrophobic patches of PTFEMA on the silica nanoparticles. Incipient flocculation of the PTFEMA-coated silica nanoparticles produces PTFEMA/silica aggregates. TFEMA diffuses from the giant monomer-droplets into these nascent nuclei, which become monomer-swollen and grow in size.
PTFEMA/silica nanocomposite particles are produced with a well-defined core–shell morphology. During the aqueous emulsion polymerization of TFEMA in the presence of a 19 nm diameter glycerol-functionalized silica sol using a cationic azo initiator at 60 °C when targeting 10% w/w solids. The onset of particle nucleation is indicated by the blue arrow. Scattering patterns are scaled by an arbitrary factor to avoid overlap and improve clarity. Postmortem TEM image of the final PTFEMA/silica nanoparticles showing well-defined core–shell nanocomposites. According to Figure Figure3 3, Interval I lies between 0 and 11 min.
During this time period, 1H NMR analysis indicates a discernible increase in the rate of polymerization, suggesting that particle nucleation occurs within this time frame. Furthermore, Interval I typically exists up to 10–20% monomer conversion2 and, according to the data presented in Figure Figure4 4b, the end of Interval I corresponds to approximately 20% TFEMA conversion. Inspecting Figure Figure3 3, Interval II is complete after around 28 min, which is consistent with the period of rapid polymerization observed by 1H NMR spectroscopy over this time period. According to the literature, the typical monomer conversion at the end of Interval II is around 60%.2 However, for the specific formulation studied herein, the TFEMA conversion at the end of Interval II is approximately 84%. After this time point, 1H NMR indicates a reduction in the rate of reaction until the TFEMA polymerization is more or less complete after 60 min. This matches the concomitant reduction in solution conductivity—and hence the beginning of Interval III—observed in Figure Figure3 3.
Furthermore, DLS studies indicate a significant reduction in the rate of particle growth over the second half of the polymerization. TEM images recorded for PTFEMA latex particles prepared during the aqueous emulsion polymerization of TFEMA at 60 °C in the absence or presence of SDS surfactant. Images a–d correspond to PTFEMA nanoparticles formed during the laboratory-scale synthesis. Images e and f correspond to post-mortem analysis of PTFEMA nanoparticles formed during the in situ SAXS synthesis using the stirrable reaction cell.
The first goal of this work was the preparation of a water-in-oil microemulsion from components generally regarded as safe for use in humans. Stable formulations without need of a co-surfactant were prepared from isopropyl myristate , dioctyl sodium sulfosuccinate , and water. A ternary phase diagram was prepared for the IPM/DOSS/water system. The IPM/DOSS/water microemulsions were characterized by conductivity and dynamic laser light scattering . The results obtained from conductivity experiments indicate conductivity values of less than 1 muS/cm and were consistent with the formation of w/o microemulsions. The DLS results showed that the emulsified water droplets had an average diameter range of 9.2 to 19.7 nm, depending on composition.
Modulation of the droplet size is possible by varying the water to DOSS molar ratio and DOSS to IPM ratio. The second goal of this work was the preparation of silver sulfadiazine nanoparticles. It was hypothesized that two separate microemulsions containing dispersed aqueous droplets of either sodium sulfadiazine or silver nitrate would react when mixed.
The DLS results are consistent with the successful formation of submicron AgSD crystals. SAXS patterns recorded in situ during the aqueous emulsion polymerization of TFEMA at 60 °C targeting 5% w/w solids under surfactant-free conditions and in the presence of SDS surfactant. Representative fits for scattering patterns recorded at specific time points for both formulations with data fits represented by either yellow (surfactant-free formulation) or green lines, respectively. All scattering patterns are scaled by an arbitrary factor to avoid overlap and improve clarity. Evolution from silica nanoparticles in co-existence with monomer droplets to micellar nucleation and subsequent particle growth. The cell volume is approximately 2.0 mL, which is sufficient to enable postmortem analysis of the final core–shell nanocomposite particles using 1H NMR spectroscopy, DLS, TEM and TGA.
A one‐step strategy to fabricate magnetically stirrable microparticles with geometric/chemical anisotropies via a microfluidic technique combined with partial phase separation is presented. Monodisperse oil‐in‐water microemulsions composed of magnetite nanoparticles and two polymers, polystyrene and poly(d,l‐lactide‐co‐glycolide) , dissolved in chloroform are generated using the microfluidic method. Upon incubating the microemulsions in pure water at ambient conditions, the solvent contained in the microemulsions is gradually removed and partial phase separation between the two polymers occurs spontaneously. In the meantime, the microemulsion droplets are vertically aligned due to the density difference of the two polymer phases. During the spontaneous phase separation, the MNPs become unstable and the aggregated MNPs segregate downward by gravity to the denser PLGA phase. After complete removal of the solvent, the resulting particles adopt geometric/chemical anisotropies, and they are magnetically rotatable under an external magnetic field.
It is demonstrated that the morphology of the anisotropic particles can be controlled readily by adjusting the ratio of the two polymers as well as the concentration of MNPs. It is believed that the developed method based on the partial phase separation and the gravity‐induced segregation of the MNPs enables large‐scale production of magnetically stirrable microparticles. A single‐step strategy is developed to fabricate magnetically stirrable particles with geometric and chemical anisotropies via a microfluidic technique combined with partial phase separation. The morphology of the anisotropic particles can be controlled readily by adjusting the ratio of the two polymers as well as the concentration of the magnetic nanoparticles. Oxalyl chloride (8.15 mL) is added dropwise to the cold mixture (10±5° C.) of Thiazole acid 8 (20.18 g) is dissolved in THF and DMF (300 μL) over a period of ˜5 min keeping the internal temperature at 10±5° C.
The cooling bath is removed and the mixture is allowed to reach ambient temperature over a period of ˜30 min. The mixture is stirred at ambient temperature for 30 min to 1 hour. A solution of aniline 7 (19.8 g), DMAP and THF was added at 10±5° C.
The mixture was allowed to reach ambient temperature, diluted with EtOAc and washed with water . NaHCO3(5%, 225 mL) was added to the organic portion and the mixture was stirred at ambient temperature for 30 min. The organic portion was concentrated under reduced pressure at approx. EtOAc was added to the resulting material and the residual water was removed and the mixture was concentrated under reduced pressure at approx.
How To Calculate Minimum Volume EtOAc was added and the resulting slurry was stirred for 2-6 h and filtered. The solid was washed with EtOAc followed by heptane and air dried for 1 h to give the desired product in 70% yield. 96% TFEMA conversion, with a final volume-average particle diameter of 244 nm and a DLS polydispersity of 0.03).
Furthermore, TEM analysis confirms the formation of well-defined core–shell PTFEMA/silica nanocomposite particles with a number-average particle diameter of approximately 215 nm (see Fig. 5b). The variation in contrast for the nanocomposite particles indicated by TEM studies in Fig. 3b–d and 5b simply reflects the significantly greater particle volume in the latter case, which attenuates the high-energy electron beam more effectively. We present a simple method with the aid of a microfluidic droplet-generation technique to fabricate magnetic Janus particles by utilizing a solvent evaporation-induced phase separation and preferential partitioning of magnetic nanoparticles in the polymer blends. Non-aqueous emulsion droplets of the polymer blends and nanoparticles solution are produced to become Janus particles after the evaporation of the solvent. The stabilizing polymer of the nanoparticles, which is compatible only with one of the polymer blends to be phase-separated, plays a key role in the anisotropic positioning of the nanoparticles in the Janus particles.
Using this phase separation-based method and microfluidics, excellent control over the size, size distribution, and morphology of the particles is achieved. Especially, we could control the morphology of the Janus particles easily by varying the volume ratio of the polymers. However, with an analysis of the shapes of resulting Janus particles, we found that non-equilibrium aspects of the evaporation-induced phase separation play a major role in determining the particle morphology.